What is the purpose of the study?
Knee osteoarthritis is a major problem in Australia and there is no cure for the disease. Non-drug treatments that help people to self-manage the condition are needed. Osteoarthritis affects the outer (lateral) compartment of the knee in a small a but significant subset of people. Unfortunately, there has been little research to evaluate which treatments can be beneficial in people with lateral compartment osteoarthritis. Footwear is an option with great potential. Different types of shoes influence forces acting across the knee joint. We know that increased knee forces can contribute to the knee pain associated with knee osteoarthritis, and that high forces can increase the risk of the disease worsening over time. It is recommended that clinicians provide advice on “appropriate” footwear for people with knee osteoarthritis. However, there is little evidence from clinical trials to determine which shoes are best for self-managing knee osteoarthritis.
We are conducting a research study to compare the effects of two types of readily available off-the-shelf walking shoes on knee osteoarthritis symptoms in people with osteoarthritis in the outer (lateral) compartment of the knee joint. To do this, we will allocate people via a random process into two different groups. Participants in each group will be provided with a pair of study shoes to wear daily for 6 months. To ensure that this is a fair and unbiased evaluation, we will not disclose the differences in the shoe types between the two groups until the end of the study. There will be an equal number of participants in each group, and participants will not be able to choose which group they are in.
Who can participate?
You can participate in the study if you:
- are aged over 50 years,
- have mild to severe knee osteoarthritis on x-ray (in the outer knee compartment) and
- currently have knee pain on most days.
You are not eligible if you:
- have arthritis on x-ray predominantly in the inner compartment of your knee,
- have had an injection into your knee in the past 3 months;
- have had surgery for your knee in the past 3 months;
- plan to have an injection or surgery within the next 6 months,
- you have a systemic arthritic condition,
- have had a knee or hip joint replacement or high tibial osteotomy on your most painful knee/side,
- have other muscular, joint or neuromuscular condition that affects your walking,
- you currently use prescribed shoe insoles,
- currently use a gait aid such as a walking stick, or
- have a significant muscular, joint or neurological condition in either leg
What will you be asked to do?
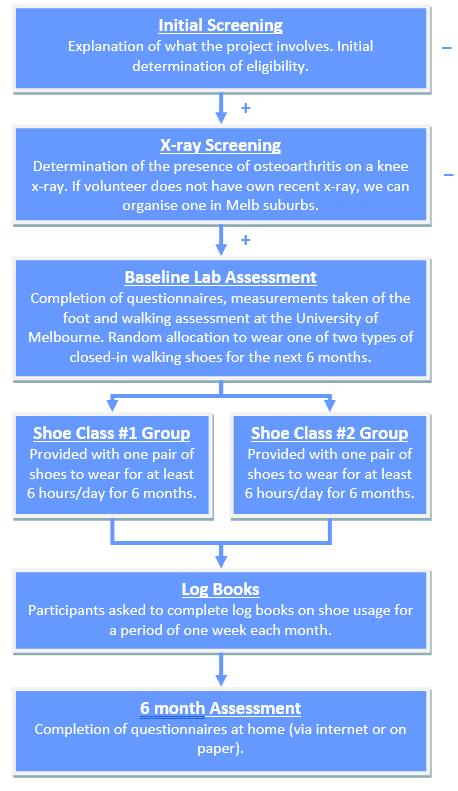
The study involves two screening steps to confirm your suitability: (1) initial screening over the internet and/or phone (which you may have already completed) and (2) x-ray screening. If you pass screening, you will be invited to take part in this 6-month study. If you decide to take part, you will be asked to sign the Consent Form (either on paper or online). You will then be allocated to receive one of two different types of footwear. This type of study is known as a “randomised trial”. To ensure the two groups in the study are similar to start with, a computer allocates each study participant into a group randomly, like the flip of a coin. Neither the researchers nor the study participant can decide which treatment the participant receives. As there are two types of shoes being compared in this study, you have equal possibility (a one in two chance) of receiving either type of shoe.
We are most interested in knowing the longer-term effects of the footwear on your knee pain and function, so we would like to ask you to commit to the research until your 6-month follow-up measures are completed. It is very important from the research that information is collected at 6 months so that we can analyse your data as part of the project.
X-ray screening
If you have not had x-rays of your knee in the past 2 years, you will firstly be asked to attend a radiology centre for a knee x-ray to determine if you are eligible for the study. These centres are located at: Bridge Road Imaging-Richmond, Blackburn South Radiology and Brunswick Diagnostic Imaging. You may attend the centre that is most convenient to you. The x-ray will take around 15 minutes and involves a small amount of radiation. There is no cost to you for this x-ray.
If you have a suitable x-ray of your knee taken within the past 2 years, the researchers will send you a stamped addressed envelope to send the x-rays in to the University for an assessment. Once this has been done the researchers will send the x-rays back to you.
Laboratory assessment
We will call you when we have the x-ray results. If the x-ray shows you have knee osteoarthritis predominantly on the outer side of your knee you will be eligible to take part in the study. If you are deemed suitable to take part, you will then attend the University of Melbourne, Department of Physiotherapy Human Movement Laboratory to undergo the baseline assessment, which will take between 1.5 to 2 hours. It will involve completing a set of questionnaires which ask about your personal details, knee pain and function, your medications usage, previous knee treatments, physical activity levels and quality of life.
Foot assessment
One of the researchers will also perform some assessments of the posture and mobility of your feet while you stand barefoot in a normal stance.
Shoe assessment
The researchers will ask you to bring with you to the University appointment your 3 most commonly worn pairs of shoes. The shoes will be assessed for style and structural characteristics. You can take your shoes home with you afterwards.
Group allocation
You will then be randomly allocated to receive one of two different types of shoes using a computer program. You have a 50% chance of being allocated to either group. Both types of shoes are readily available off-the-shelf shoes, rather than medical/orthotic shoes designed specifically for medical conditions. You will be provided with a pair of shoes in your size and will be asked to wear these shoes daily for the next 6 months for at least 6 hours per day.
To ensure that this is a fair and unbiased evaluation, we will not disclose the differences in the shoe classes between the two groups until the end of the study. We would prefer you to avoid starting any treatments for your knee pain if possible during the study. However, you can continue to take any regular medications you may be using. If you do undergo new treatments or begin taking new medication, you will have the opportunity to report these to the researchers in the questionnaire at the 6-month mark.
Walking assessments
We will conduct two types of walking tests. The first will assess your walking patterns, (1) with barefeet, (2) and with the shoes you have been allocated. As part of the walking assessment, you will be asked to change into shorts. Reflective skin markers will be placed over your skin at various sites such as the ankle, knee and hip using adhesive tape. The markers on your skin will be tracked as you walk along a 10-metre walkway 6 times at your normal walking pace for each of the 3 conditions. You will have a rest in between each condition and are free to take further rests from walking if you would like them. From these tests, we will be able to analyse the movements at your hip, knee and ankle, as well as the forces acting across each joint. We will ask you to rate the pain felt in your knee during these walking tests. We will also ask you to rate the comfort of the shoes.
For the second walking assessment you will be asked to walk in (1) your most commonly worn pair of your own shoes, (2) and with the shoes you have been allocated. The walking assessment will involve putting some thin insoles inside the shoes that have pressure sensors inside them, which are attached to a belt worn around the waist. You will be asked to perform 6 walks of an 8-metre walkway at your usual pace in each of the 2 pairs of shoes (for 12 walking trials in total).
Log-books
Over the 6 months, you will be asked to complete a short log book for one week each month. In the log book you will indicate how many hours you wore the study shoes for each day of that week. The log books will be provided with a stamped addressed envelope to return them in the mail.
Follow-up
Six months after being given your study shoes, you will be sent a follow-up questionnaire to complete at home (either online or on paper where we will provide a stamped addressed envelope). These questionnaires will be the same as those completed at baseline about level of knee pain and symptoms, medications usage, physical activity levels, quality of life. You will also answer questions about any adverse effects you experienced.
Debriefing
Following the completion of the entire study, you will be contacted by email or mail to be informed of the type of shoes that you were provided with, and information about the specific hypotheses the study was testing, as well as the main study findings.
Will my details be kept confidential?
No findings that could identify you will be published. All data and results will be handled in a strictly confidential manner. This project is subject to the requirements of the Human Research Ethics Committee of the University of Melbourne (HREC No. 1852787.1).
What to do next?
If you would like to participate in this project, please click the 'next' button below to submit your details and complete the screening questionnaire and we will contact you as soon as we can. Or you can phone Penny Campbell on (03) 9035 5702.
About the researchers:
Prof Rana Hinman is a research physiotherapist who has conducted 20 randomised controlled trials in musculoskeletal conditions, most in knee osteoarthritis. She is the Chief Investigator of this trial.
Prof Kim Bennell is a research physiotherapist and Director of the Centre for Health, Exercise and Sports Medicine (CHESM).
Dr Kade Paterson is a podiatrist and musculoskeletal researcher at the Department of Physiotherapy at the University of Melbourne.
Mr Tim Wrigley is a biomechanist and technologist whose career has been based on research and development for human movement analysis, in academia and with industry.
Ms Penny Campbell is a Research Scientist, who will take on the role of Trial Co-ordinator. This will include participant recruitment, administration of questionnaires and scheduling of participant appointments.
Dr Jessica Kasza is a biostatistician from the Department of Epidemiology and Preventative Medicine at Monash University.
Mr Ben Metcalf is a Research Scientist, who will help develop the study protocol and study documents. He will also collect laboratory baseline data, randomize participants to shoe group and fit allocated shoes.